Unique emphasis really should be put over the management of your constitutive excipients in the formulated active material. Technical specs must be outlined for excipients Based on GMP Part I., four.fourteen and the monographs of the European Pharmacopoeia ought to be used. The acceptance, routine maintenance and audit of excipient suppliers should be determined by quality hazard administration, in accordance with GMP Part I, 5.
Technological agreements are part of the EU PQR as each a necessity for review in order that these agreements keep on being up to date and a essential doc among the internet marketing authorization holder (MAH) (i.e., the product-license holder) plus the company where these are generally distinctive parties. The goal of the technological agreement, inside the latter instance, should be to outline the tasks involving the two parties in developing and reviewing the PQR. The technical agreement, occasionally called a quality arrangement inside the US, has an extended-standing position inside of European GMPs and is roofed as the main subject matter of Chapter seven with the EU GMP guidebook. Both equally the EU as well as the US business objected towards the requirement for a particular specialized arrangement masking PQR among a MAH as well as a manufacturer. PDA, within their reviews to EMEA, stated the draft need to get a technical arrangement among a MAH plus a producer "exceeded requirements in other marketplaces and added sizeable administrative burdens with unclear benefit"(thirteen). The EFPIA pointed out that "a worldwide business may have around 100 distinctive MAHs either as affiliates to the corporate or as license holders or agents, Which these firms do not have usage of the data or even the skills required to perform the review or Examine the information"(fourteen).
When outsourced, the manufacture of a formulated Lively substance need to be managed in exactly the same way because the outsourcing from the manufacture of an intermediate medicinal product, via entire application of the necessities of Chapter 7 from the GMP portion I guideline.
Knowledge must be accessible to the PQR manager all of the time and should be confirmed by a next particular person if gathered manually.
Being able to weld and knowledge welding are two various things. Another person that includes a tested means to be aware of what has an effect on the end result will always be a better option.
Nationwide skilled authorities need to be notified of all remember action proposed once the product has become placed out there. In situations where by the MAH can exhibit which the batch is reconciled without having issuing a remember see, the countrywide proficient authority might agree that public remember conversation through the entire distribution network is not necessary.
The MIA holder to blame for QP certification should have usage of most of the contracts in the “chain of contracts”. Deal companies ought to have usage of These contracts within the “chain of contracts” pertinent on the functions they perform along with the associated tasks.
Ought to a company of the medicinal gasoline receive a serious grievance associated with the quality of the medicinal gas by itself or even the packaging elements, the system set up must enable the identification from the affected cylinders and, in which necessary, the remember of any impacted cylinders from the market.
The registered specs of our starting components contain regular or pharmacopoeial solutions with the confirmation of id but we would like to use NIR to accomplish identification tests on Every single container of starting off elements Utilized in the manufacture of parenteral products. Is the usage of this substitute process acceptable?
EU authorities are mindful that these documents are also used to aid regulatory submissions in third nations and that several supplemental prerequisites, which include apostilled copies are occasionally predicted.
The EU PQR demands a review of your adequacy of any other previous product process or tools corrective steps. This wording was suggested in remarks provided by EFPIA to clarify the intent this portion is referring into the review of corrective steps from previous PQRs (fourteen).
This is simply not generally demanded, but it's laid out in some specifications. It may conserve heartache obtain making certain the customer is satisfied with the WPS at the end.
Inside the text of ICH Q10, “Administration review should really present assurance that course of action overall performance and product quality are managed over the lifecycle”.
The method by itself really should be designed to comply with the registered necessities (in shape for intent). A deviation may be regarded as 'unanticipated' till some time of discovery. Exactly where the related authorities have verified the need to here keep away from provide disruption, repeat deviations thereafter are now not 'unanticipated' but could be considered for QP certification and recognized although corrective and preventive action is in progress and in which get more info the provisions of Annex sixteen paragraph three.1 are met.
 Devin Ratray Then & Now!
Devin Ratray Then & Now! Romeo Miller Then & Now!
Romeo Miller Then & Now!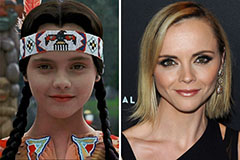 Christina Ricci Then & Now!
Christina Ricci Then & Now! David Faustino Then & Now!
David Faustino Then & Now! Barbi Benton Then & Now!
Barbi Benton Then & Now!